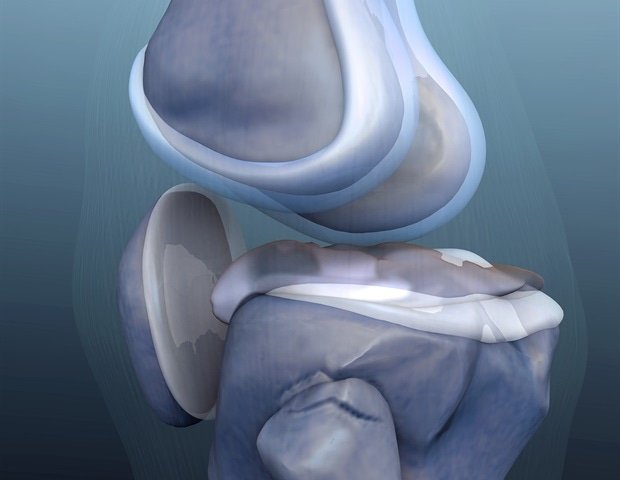
The Exactech Medical Evidian manufacturer agreed to pay $ 8 million to settle allegations that it had hidden defects in a popular line of artificial knee implants, which were accused of thousands of patient injuries in the lawsuits.
The settlement resolves two complainant lawsuits claiming that Florida’s company has violated the federal law on false allegations, by charging government healthcare programs, such as Medicare for Parts Arpecaction, which was aware.
“Patients who need a medical device to enjoy their lives are based on device manufacturers to first put patient safety. When a manufacturer learns that his device is defective, he must face the problem immediately and transparently,” said Kelly Hayes, a US lawyer for the Maryland area.
The Gainesville -based company declared bankruptcy last October, leaving thousands of injured patients in charge of compensation. The US Delaware District of bankruptcy has approved the settlement of complaints.
ExecTech refused to comment. In the production of settlement, the company did not acknowledge the responsibility.
ExecTech, which has grown more than three decades from a Mom-and-pop device manufacturer in a global entity, has been the subject of KFF Health News research published in October 2023.
Florida’s lawyer Joseph Saunders, who represents patients treated for the company in other lawsuits, said more than 2,500 people who claim that injuries have filed claims seeking compensation. Many have undergone functions to remove and replace implants, some within three years of their initial surgeries. Many of these cases have been put on waiting due to bankruptcy.
“There are people who are lifelong diseases from it or have multiple surgeries,” Saunders said.
So far, patients have not received compensation. Sue Sacker, a resident of New Jersey, who had replaced two Exactech implants in the hospital for special surgery in New York, said he was “very disappointed” by the settlement agreement.
“I am smoking,” he said. “People suffer and the government gets the money. Where are the patients? Who cares for us?”
Saunders said a creditors’ committee hopes to follow TPG, a private stock company that paid $ 737 million to get Exciryek in February 2018. TPG won the dismissal of a similar effort last year when it successfully claimed that it did not successfully control it. TPG refused to comment.
The first Whistleblower case was submitted in 2018 to the Federal Court in Alabama by Brooks Wallace and Robert Farley, two former Exactech and Manuel Fuentes sales agents, former Exectech product manager.
They supported severe defects in Optetrak Total Replace replacement systems, which Exactech began selling in 1994. Until 2008, the company knew that an element of the implant “fails prematurely at higher than the acceptable interest rate”, but Exactech released implants by December 2018. Together, the complainants will receive $ 1,329,360 under the provision of the law that allows individuals to act on behalf of the government.
In the second case of the complainants, Pasquale Petrera, an orthopedic surgeon of Maryland, claimed that since January 2019, Excome knew that a polyethylene section in certain logic logic and brand knee replacement systems failed prematurely. The company sold them until the beginning of February 2022, when it expanded the recall of the product. Maryland’s whistleblower will receive $ 565,360, according to the settlement amount.
KFF Health News found and reported in 2023 about 400 examples in which Excome reported adverse reactions to the government two years or more after learning them. Reports are supposed to be submitted within 30 days, unless special discharge is granted.
The News Health KFF analysis for more than 300 pending cases in Florida’s Alachua province found that surgeons were removed about 200 knee and hip implants after less than seven years, much earlier than 15 to 20 years these products usually last. The company emphasized the durability of its implants in advertising, even suggesting that they would probably overcome their human recipients.
In court deposits, Exactech has steadily denied that Optetrak had defects.