• Research Highlights
People with sensory hypersensitivity have a heightened awareness and reactivity to sensory stimuli, such as sound, sight, touch, and taste. Sensory hypersensitivity is a symptom often associated with Autism Spectrum Disorder (ASD), as over 90% of children with ASD experience this sensory challenge.
Researchers examining the neural underpinnings of sensory hypersensitivity have often focused on the role of the forebrain—an area at the front of the brain involved in processing sensory stimuli and regulating behaviors and emotions. However, new research suggests that brain circuits that support sensory reflexes—which occur much earlier in sensory processing—could also contribute to this sensitivity.
What did the researchers do?
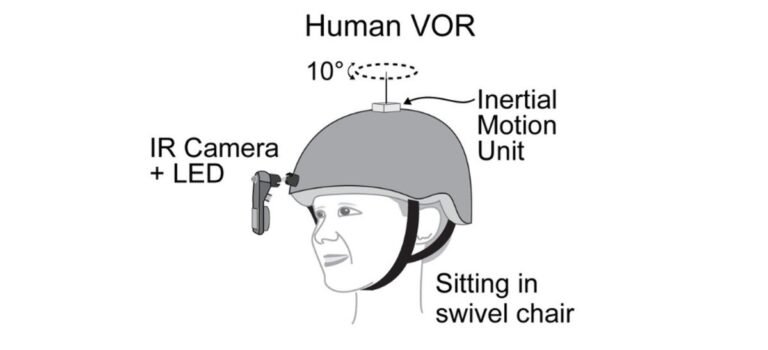
To learn more about the neural underpinnings of sensory hypersensitivity, a new NIMH-funded study by Chenyu Wang, Ph.D., a researcher at the University of California, San Francisco, and colleagues looked at regions of the brain that control the vestibular -ocular reflex. This reflex stabilizes the images on our retina, causing our eyes to move in the opposite direction of our head.
The researchers looked at how the brain controls this reflex in humans and mice with a genetic mutation called SCN2A loss of function. SCN2A Loss of function has the strongest association with ASD of any known genetic mutation. The SCN2A The mutation affects the way neurons move charged particles, called ions, in and out of the cell. This, in turn, affects how the cells (including those involved with the vestibulo-ocular reflex) communicate electrically with each other.
Participants included children between the ages of 3 and 10 with the SCN2A loss of function genetic mutation diagnosed with ASD, as well as children of a similar age who did not have the mutation or were diagnosed with ASD. All participants wore a lightweight helmet that tracked their eye and head movements. The researchers found that the vestibulo-ocular reflex was hypersensitive in children with SCN2A loss of function genetic mutation compared to those without the mutation. The researchers found that mice with the Scn2a The loss-of-function mutant also showed this pattern of increased vestibulo-ocular reflex behavior.
To find out what might be causing this pattern of vestibular-ocular reflex behavior, the researchers recorded electrical activity in the brains of mice with Scn2a loss of function mutation. They found that activity among cells involved in supporting the vestibulo-ocular reflex (called Purkinje and cerebellar granule cells) did not change in response to different levels of speed and head movement, unlike what would occur in mice without this genetic mutation. This lack of adaptation of cellular activity was found to be driven by the Scn2a loss of function mutation in granulosa cells.
To see if a typical vestibulo-ocular reflex could be restored, the researchers administered a gene therapy to mice that targeted Sn2a loss of function mutation when it was either 3 days old or 30 days old. The researchers were able to fully restore typical vestibular-ocular reflex behavior in mice treated at 3 days of age, but restoration was only partial in mice treated at 30 days of age, suggesting that there is an early developmental period that enhances aspects of this reflex.
What do the results mean?
This study sheds light on the neural processes involved in a specific symptom associated with ASD – sensitivity to sensory stimuli. Furthermore, since the vestibulo-ocular reflex was hyperactive in children with SCN2A autism-related loss of function, the findings suggest that an ocular reflex test could be used in the future as an early diagnosis of ASD. Furthermore, a gene therapy restored typical vestibular-ocular reflex behavior when administered to very young mice with Scn2a loss of functionality, suggesting a possible way to improve sensorimotor dysfunction in the future. The study also suggests that cerebellum-dependent reflexes, such as the vestibulo-ocular reflex, may prove to be generalizable and translatable markers for determining the effectiveness of potential treatments aimed at addressing the sensorimotor symptoms commonly experienced by individuals with ASD.
Report
Wang, C., Derderian, KD, Hamada, E., Zhou, X., Nelson, AD, Kyoung, H., Ahituv, N., Bouvier, G., & Bender, KJ (2024). Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-related ASD. Neuron. https://doi.org/10.1016/j.neuron.2024.01.029