In a recent research letter published in the journal Aging Nature, researchers used proteomic sequencing of the cerebrospinal fluid of patients and controls to explore the heterogeneity of Alzheimer’s disease. Their findings revealed five molecular subtypes that depicted distinct genetic risk factors and disease pathologies, including rates of progression and survival time. These results suggest different intervention requirements for each subtype and highlight the need for personalized medicine to diagnose and treat the condition.
Letter: Cerebrospinal fluid proteomics in Alzheimer’s disease patients reveals five molecular subtypes with distinct genetic risk profiles. Image credit: Lightspring / Shutterstock
Alzheimer’s disease and the advantages of proteomics
Alzheimer’s disease (AD) is a progressive brain disorder that mainly affects elderly people, characterized by the degeneration of neurons responsible for memory and cognition. It is estimated to affect 5% of people aged between 65-74, 13.1% between 75-84 and 33.3% over 84, currently affecting 44 million people, with this number increasing annually . AD is recognized as the leading cause of dementia worldwide, with no cure currently known and treatment limited to symptom management. While a definitive substrate for the disease has yet to be identified, genetics and environmental exposure are thought to be responsible for the condition.
Recent research has identified that AD is not a single disease but an umbrella term for a spectrum of conditions that vary significantly at the molecular level. Unfortunately, these research developments invalidate much of the previous literature attempting to elucidate the clinical pathophysiology of AD, since different patients may respond significantly differently to the same clinical exposure.
“Proteomics” is the study of the interactions, function, composition and structures of proteins and their cellular activities. It incorporates state-of-the-art “next-generation” sequencing techniques such as mass spectrometry (MS) to identify and characterize thousands of protein subunits in biofluids. Cerebrospinal fluid (CSF) is the most accessible of these biofluids associated with neurological diseases due to its constant contact with the brain and central nervous system (CNS) and its role as a proxy for the pathophysiological process of the brain.
About the study
In the present study, the researchers used a case-control cohort approach, using CSF from AD patients and age-matched healthy controls, to reveal the differentially up- and down-regulated proteins in these cohorts through proteomic analyses. The study sample group came from the Amsterdam Dementia Cohort (ADC), an ongoing study of all patients who sought treatment at the Alzheimer Center in Amsterdam since 2000.
Study inclusion criteria included diagnosed AD, confirmed based on the presence of an abnormal amyloid marker (cases), and age, sex, and demographically matched controls. CSF from both groups was collected and subjected to high performance liquid chromatography (HPLC) mass spectrometry (MS) – LC-MS/MS. Enzyme-linked immunosorbent assays (ELISAs) were then used to measure amyloid-β42t-tau, p-tau 181, and amyloid-β42/amyloid-β40 ratio, the main determinants of AD severity and stage of progression.
Blood samples from cases and controls were further subjected to apolipoprotein E (APO) genotyping to screen for single nucleotide polymorphisms known to enhance or suppress AD. T1-weighted magnetic resonance imaging (MRI) was used to visualize brain atrophy patterns and assess differences in neuroimaging of AD patients and controls. Finally, standardized neuropsychological test batteries were administered to study subjects at initial enrollment, with annual follow-up to assess rate and degree of AD progression.
Study findings
The present study included 609 cases and 187 controls. Of the included AD cases, 107 had normal cognitive function, 103 had mild cognitive impairment (MCI), and 209 had dementia. LC-MS/MS analyzes identified 3,863 unique CSF proteins, of which 1,309 proteins (28,408 peptides) were common to all included participants and used for further analyses. Of these, cluster analyzes revealed 1,058 AD-related proteins. Combining the clustering results with the clinical characteristics of the patients revealed five distinct AD subtypes.
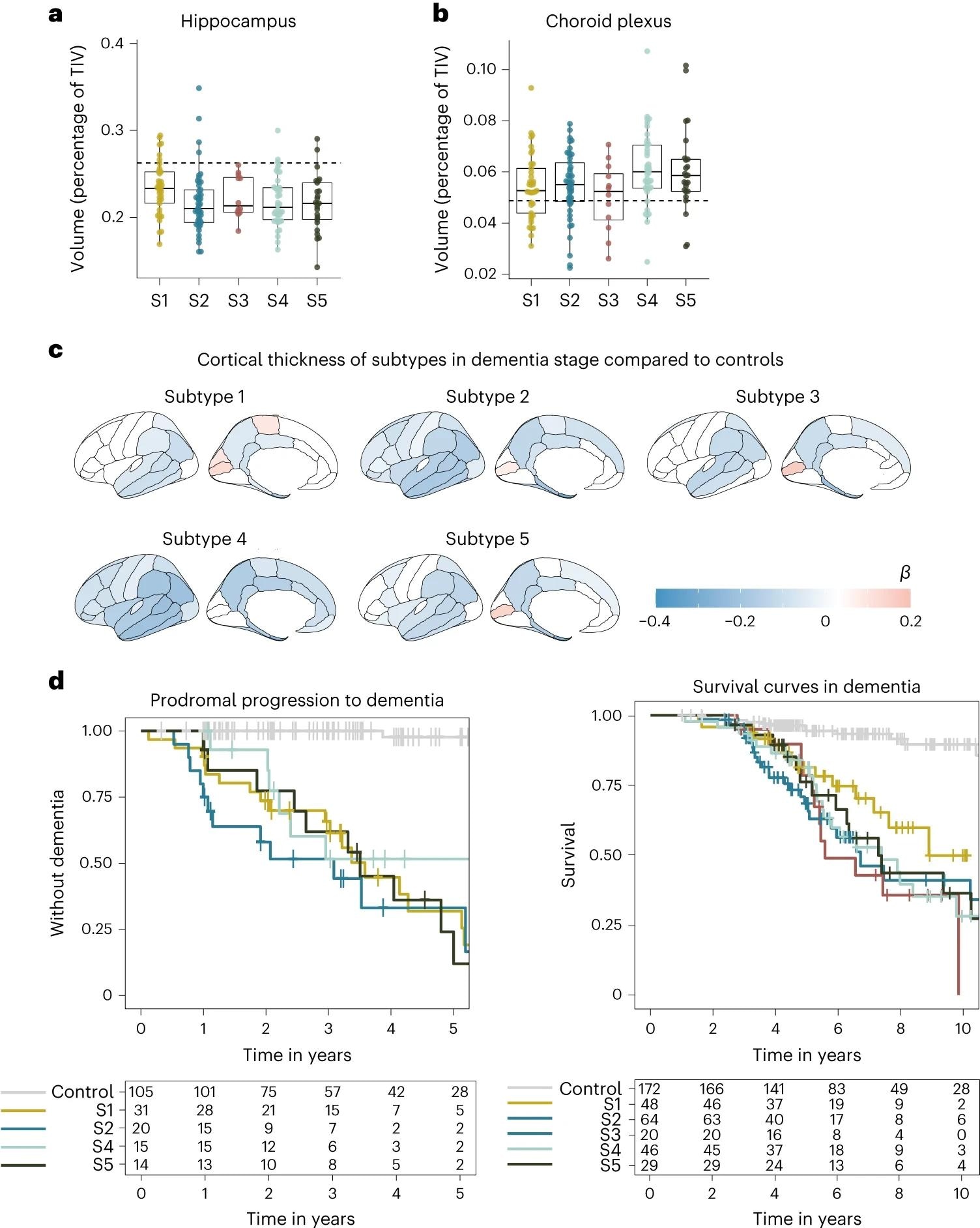 oneMedian hippocampal volume as a percentage of total intracranial volume (TIV) compared to subtypes in dementia stage. siChoroid plexus volume as percentage of TIV compared to subtypes in dementia stage. do, Cortical atrophy associated with AD subtypes in the dementia stage compared to controls (n = 160). b shows mean cortical thickness in mm, averaged over right and left hemispheres and adjusted for age and sex. Hey, Clinical progression from MCI to dementia according to subtype (left, excluding subtype 3 due to n = 2) and time from dementia to death according to subtypes (right). All measures of atrophy are based on people with dementia only. one,si, The squares depict the median at the center. limits indicate first and third quartiles, while whiskers extend up and down up to 1.5 times the interquartile range (restricted to actual observed data points), and points indicate values of individual subjects (subtype 1, n = 37, subtype 2, n = 45, subtype 3, n = 12, subtype 4, n = 40, subtype 5, n = 25).
oneMedian hippocampal volume as a percentage of total intracranial volume (TIV) compared to subtypes in dementia stage. siChoroid plexus volume as percentage of TIV compared to subtypes in dementia stage. do, Cortical atrophy associated with AD subtypes in the dementia stage compared to controls (n = 160). b shows mean cortical thickness in mm, averaged over right and left hemispheres and adjusted for age and sex. Hey, Clinical progression from MCI to dementia according to subtype (left, excluding subtype 3 due to n = 2) and time from dementia to death according to subtypes (right). All measures of atrophy are based on people with dementia only. one,si, The squares depict the median at the center. limits indicate first and third quartiles, while whiskers extend up and down up to 1.5 times the interquartile range (restricted to actual observed data points), and points indicate values of individual subjects (subtype 1, n = 37, subtype 2, n = 45, subtype 3, n = 12, subtype 4, n = 40, subtype 5, n = 25).
Subtype 1 is characterized by neuronal hyperplasticity, subtype 2 by innate immune activation, subtype 3 by RNA dysregulation, subtype 4 by choroid plexus dysfunction, and subtype 5 by blood-brain barrier dysfunction. APO Genotyping confirmed identified clusters and suggested a unique genetic background for each subtype.
In particular, we found that each subtype was associated with different genetic AD risk factors, further supporting that each AD CSF subtype reflects specific underlying molecular mechanisms.
The subtypes were found to differ significantly by their clinical pathology as highlighted by neurophysiological testing – subtype 3 was significantly more aggressive in its rate of progression compared to the other subtypes. Given the degree of genetic and pathophysiological uniqueness of these subtypes, the need for personalized medicine becomes apparent.
“…side effects resulting from certain treatments may also depend on the subtype. For example, while antibodies may more easily cross the blood-brain barrier in subtype 5, these individuals may be at increased risk for brain bleeding that can happen with antibody therapy.”
conclusions
The present study used proteomics to investigate patient-specific differences in the genetic and pathophysiological profiles under the umbrella of AD. The study findings reveal more than 1,000 proteins that are differentially expressed in AD patients, and importantly, AD comprises at least five distinct subtypes that differ in their genetic and clinical backgrounds.
“Given the distinct patterns of AD molecular processes and genetic risk profiles, it is possible that AD subtypes require specific therapies. For example, subtype 1 individuals may benefit from treatments that activate TREM2, subtype 2 from innate immune inhibitors, subtype 3 by antisense oligonucleotides that restore RNA processing, subtype 4 by inhibition of monocyte infiltration, and subtype 5 by cerebrovascular therapies’.
Journal Reference:
- Tijms, BM, Vromen, EM, Mjaavatten, O., Holstege, H., Reus, LM, Wesenhagen, KE, Lorenzini, L., Vermunt, L., Venkatraghavan, V., Tesi, N., Tomassen, J. , Den Braber, A., Goossens, J., Vanmechelen, E., Barkhof, F., Pijnenburg, YA, M., W., Teunissen, CE, Berven, FS, . . . Visser, PJ (2024). Cerebrospinal fluid proteomics in Alzheimer’s disease patients reveals five molecular subtypes with distinct genetic risk profiles. Aging Nature1-15, DOI – https://doi.org/10.1038/s43587-023-00550-7,
