Yokogawa Electric Corporation announces Oprex’s release today™ Quality Management System, a product in the OPREX functional risk management family.
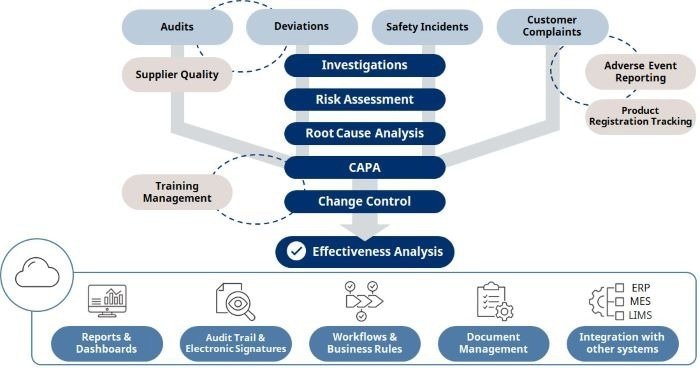
The Oprex Quality Management System is a cloud -based system that can accelerate the digital transformation of quality assurance processes for the manufacture of medicinal products and food and drinks. It means functions and allows continuous improvements, allowing monitoring and central management of quality assurance processes, such as handling deviations from production processes and specific standards, CAPA implementation, changes in manufacturing processes and revision of the documents.
Growth background
In addition to taking measures to ensure quick compliance with GMP and GQP standards and enhancing quality assurance processes, the pharmaceutical industry is trying to rationalize functions and reduce costs.
At the same time, safeguarding quality by producing medicinal products and foods and beverages involves a wide range of processes that include dealing with deviations, implementing CAPA and management of the necessary changes in construction processes, so the smooth and proper management of these processes It’s a need.
Main features
1. Construction of a Codeless Workflow for Flexible Improvements in Quality Assurance Procedures
The need to codify work flows to tackle deviations, application of CAPA, change of production processes, review of documents, creating training files and other such procedures is eliminated, allowing flexible handling without planning a wide variety of safeguarding processes. quality.
2. An advanced platform that allows to add functions and connection to other systems
This product is a system based on a cloud based on the worldwide proven, flexible and extremely scalable servicenow® platform. With this platform, updates on new features can be done quickly.
In addition, as a standard feature, various fasteners are provided for connection to construction systems, laboratory information management systems and the like. This reduces the time and effort needed to integrate data handling from different systems and the likelihood of human error, thereby allowing the accurate and effective execution of quality management work.
3. Contribution to the integrity of data through compliance with strict regulatory standards
The user control and management methods used by this system comply with global pharmaceutical regulations, leading to improved integrity in handling data for quality assurance processes. This product uses the latest safety protocols that comply with the standards of the international standards organization, thereby allowing strong security.
Important target purchases
Medicines, food and drinks, chemicals
Applications
Management of Quality Assurance Procedure