A new study reveals how GIPR signaling in brain cells helps GLP-1 weight loss drugs to bypass the bloody bloody bloody barriers and enhance the effects that destroy their appetite by offering a mechanistic explanation for clinical power.
Study: Glucose-dependent insulin-signal signaling of the polypeptide receptor in oligodendrocytes increases the weight loss action of the GLP-1R struggle. Credit Picture: Juan Gaertner / Shutterstock
In a recent study published in the magazine MetabolismA team of researchers examined whether the signaling of the insulin-to-GIPR (GLPR) (GLPR) (GLP-1R) signal (GLPR) receptor signal.
Background
One in eight adults lives with obesity and many now use barefoot drugs that can reduce weight by more than 20%. Incretins work through GIPR and GLP-1R, but because the combination helps them remain unclear. The interstitial detection (ME), a interface where blood signals meet the neurons, can be a gate.
OLS, known for the creation of myelin, also remodeles this gate in response to the diet. The clarification of whether the glucose-dependent insulin-tulip signalization of polypeptide (GIP) in OLS enhances brain input and the effects of peptide-1 (GLP-1)-like glucagon treatments. Further research is required.
For the study
Researchers used adult mice to check whether GIPR signaling to OLS shapes brain access and the effectiveness of GLP-1R agonists. Created ol Knockouts with the passage of 1-Cre Protein Protein Reform-Cre-CRE-CRET2 (PLP1-CREERT2) with GIPR mice, activated a tamoxifen reconstruction on the post-warring day 60 and caused a 60%pumping day. A long-term GIPR fighter (Lagipra) and a long-term GLP-1R fighter (Laglp-1ra, Liraglutide) were given alone or together.
To map the drug entrance, a short-acting GLP-1R fighter was injected by IR800 (IR800-Exendin-4). The brains were cleared and depicted with light microscopy. Oligodendrogenesis and myelinosis were quantified by fluorescent in situ hybridization and immunohosis for basal myelin protein, enhanced sequence of 1 carcinoma and bone protein 4, with a 5-zealos-2 ‘-phase.
Vascular permeability was evaluated by expression of vascular endothelial growth factor A (VEGF-A), Immunomonitivity of Vascular Endothelial Growth (VEGF) and Antigone Endothelial Cell 32 (MECA32) glucose and insulin.
Finally, an adenom-related virus that encodes the inhibitory human muscarinus receptor M4 designed to activate the designer Druger (AVPI) neurons (AVP-CRE). Deschloroclozapine was conducted to activate HM4DI and suppress these neurons during Liraglutide tests.
Results
In Me, GIPR was enriched in mature Ols, with rare expression in the ancestral cells OL. High fat supply has increased the density of OL and the number of positive GIPR OLS especially in this area. This effect was not observed in white matter such as Corpus Callosum, indicating a local role.
The GIPR specialization for OL reduced oligodrogenesis of adults and the survival of OL in ME and reduced the basic myelin protein, while large white matter areas showed minimal changes, indicating a local role in the metabolic gate of the brain. Mice lacking OL GIPR showed reduced energy costs and intake, maintained tolerance from the glucose mouth, reduced insulin tolerance, and metabolites to branched chain metabolites, according to altered substrate handling during obesity.
Pharmacological activation produced complementary results. In lean mice, a long -acting GIPR agonist increased the cells of the OL line and the myelin in the ME. In obesity caused by diet (DIO), the same militant increased the new OL production and restored turnover, while increasing vascular access signals: VEGF-A transcripts and VEGF immunomodicity increased and MECA32 marked by the haircut. vases.
The pre-installation of obese mice with GIPR agonist has increased the brain of a GLP-1R agonist highlighted by IR800 on the ME and neighboring arched core of the hypothalamus (ARH), indicating improved access to the ME-ARH border. Basically, this increase in intake required OL GIPR. It is absent after the deletion of Ol Gipr.
Effectiveness reflects the entrance, as in wild mice, the long-term GLP-1R action has reduced food intake and body weight and co-infrony with the GIPR agonist reinforced both results. In Ol Gipr Knockouts, GIPR agonist no longer enhanced weight loss or anorexia driven by GLP-1R, indicating that the signaling of OL GIPR is required for complete synergy.
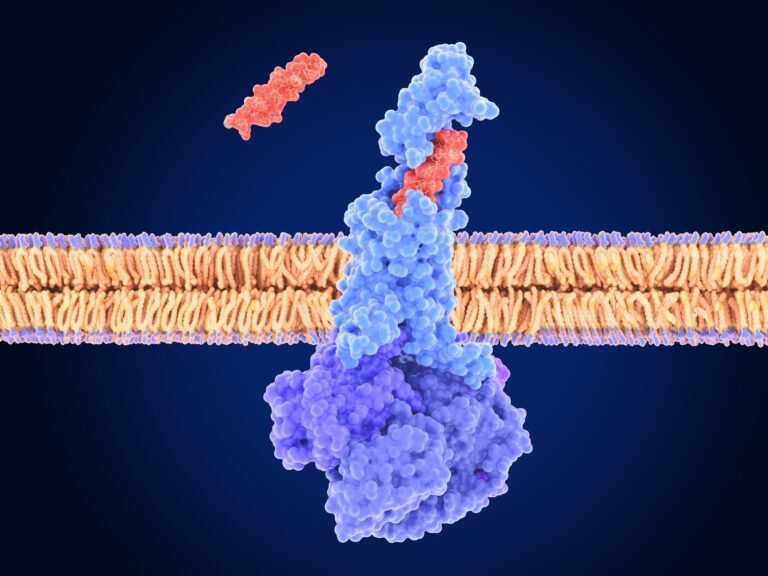
Imaging showed that GLP-1R agonists who had been dosing peripherally doses GLP-1R short circuit accumulated along the myelized axis packages in ME, accumulated with basal myelin protein. Revealing a new mechanism: GLP-1R fighters who are administered peripherally access to the brain through myelized AVP neurons to ME, bypassing the blood-brain barrier (BBB).
Hyper-analysis microscopy was detected GLP-1R on AVP shafts and nodes marked with contactin-related protein (Caspr). Finally, the chemogenetic mute of the AVP PVH neurons with adhelorosapine prevented the caused by laraglidal subcontinent and weight loss, proving that these neurons are essential for the weight loss action of the systemic drug.
Conclusions
In summary, this study connects the pharmacology of the prostitute to a concrete brain input mechanism: GIPRS signaling to Me Ols increases vascular permeability through VEGF-A induction and increased capillary moonlight and allows GLP-1R agonists.
The requirement of this route can help explain why coipr/GLP-1R co-agonists show greater efficiency than individual factors and offer a mechanistic basis for their enhanced clinical performance. Clinically, the mechanism helps to interpret the power of co-authors used for obesity and type 2 diabetes and shows biomarkers, such as induction or visualization of VEGF-A or visualizing my access, guidance of dosage or combinations while limiting side effects.
However, the authors note several restrictions: Knockout model OL GIPR only achieved partial deletion. The experiments were primarily evaluated by Ligoutidia and not the other GLP-1R fighters. And the effects of behavior, though informative, were not exhaustive. These warnings mitigate the conclusions and emphasize the need for further investigation to confirm generalization and clinical significance.
Why does Zepbound (Tirzepatide) lead to greater weight loss than ozempic (semi -blister)?
Double receptor (GLP-1+ GIP) has more intense results in the brain pic.twitter.com/ws81pf5cxf– Eric Topol (@erictopol) August 13th 2025
Magazine report:
- Hansford, R., Buller, S., Tsang, Ah, Benoit, S., Roberts, Ag, Erskine, E., Brown, T., Pirro, V., Reimann, F., Harada, N., Inagaki, N., Samms, RJ, Broichhagen, J., Hodson, DJ, DJ, Adriaenssens, A., S. Dependent of glucose insulin-digestion signaling of polypeptide receptor in oligodendrocytes increases the weight loss action of the GLP-1R race. Metabolism. DOI: 10.1016/J.CMET.2025.07.009,