Did you know that research has shown that the risk of developing type 2 diabetes is almost 10 times higher for women who had gestational diabetes during pregnancy (compared to women who did not have gestational diabetes)?
The SWEET The study is a research study to find out if a once-weekly drug called Ozempic can help women get rid of prediabetes and have healthy blood sugar levels after a pregnancy with gestational diabetes.
SWEET Study participant Erica shares her SWEET study journey in hopes that those who qualify can follow her on a health journey. Learn more about the study or see if you qualify by clicking here.
Week of April 1st – Meet Erica!
So, hello! My name is Erica and I am participating in the SWEET Study at Woman’s Hospital.
What is SWEET Study, you ask?
In layman’s terms, this is a study of women with prediabetes who have recently had gestational diabetes to see if a drug called semaglutide (Ozempic) can normalize blood sugars and prevent type 2 diabetes. The study lasts up to eight months to complete and is tracking some women on active Ozempic and some on placebo. The fun part is that neither the test subjects nor the research team will know whether the participant will receive the placebo. In the end, the researchers will compare the results of the two test groups to show whether Ozempic is successful in controlling blood sugar in this particular group of women.
I received my first batch of injections on Monday March 20th!
Follow me to learn all about my experience with this test and how my journey is going.
Let the adventure begin!
Week of April 10 – The SWEET Shows
Let’s start from the beginning.
How did I find out about the study and the screening process?
The first qualification to participate in the SWEET study is to have had gestational diabetes during at least one pregnancy. I had it in both my pregnancies and was prescribed metformin to control my insulin levels. I wouldn’t call either of my pregnancies fun, but it was worth it for my beautiful family.
The first time I heard about this study was through an email from Women’s Hospital. I’ve signed up for survey opportunity alerts in the past, hoping to make some extra cash. When I read the study description and the required criteria that participants had to meet, it described the perfect opportunity for me to help make a difference — and of course, the extra cash didn’t hurt.
Another qualification to participate in the study is that you cannot breastfeed. When I first learned about the study in July 2021, I was still exclusively pumping for my son Ezra, so the first screening call was over quickly.
On January 23, 2022, I spoke with Bri on the research team and we arranged my first screening visit. I came off metformin, which took four weeks to “wash out”. I am currently on birth control and Effexor, both of which are approved to be taken while participating in the study. In fact, birth control is very important as they don’t know the effect Ozempic has on pregnancy.
Screenings:
February 20, 2022 – Initial check by phone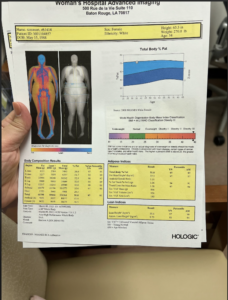
Bri provided me with the informed consent form, which is a complete breakdown of how each visit will go and what will be expected of me throughout the study. In addition, he also shared the benefits that the results of this study could provide. Then I started thinking about why I should be a test subject for this research.
Facts I Learned About Gestational Diabetes:
- Women who develop gestational diabetes have a much higher chance of developing type 2 diabetes.
- This chance increases with each affected pregnancy.
Objectives of the researchers:
- The researchers hope to show that Ozempic can help maintain healthy sugar levels in these mothers.
My fears about the trial:
- Worries about whether I will be able to be consistent and stick with it for that long.
- I don’t want to disappoint anyone or stretch myself too much.
- I had also heard about some of the difficult side effects of Ozempic and was worried about how it would affect my ability to function as a mother and employee to my full potential.
Finally, I prayed hard about it and felt that this was an opportunity for me and many mothers to become the best version of ourselves!
My hopes for the trial:
- The possibility of having extra help to control my health, as well as making extra cash, was the biggest incentive to join.
- This is my chance to do something for myself. I want to be healthy and feel good about enjoying this life with my kids!
- Helping mothers live longer, healthier, more active lives for their children was an opportunity I felt blessed to be a part of.
I couldn’t care less what size clothes I’m in, because let’s be honest: I’m fine no matter what. But I’m tired, sore, and probably couldn’t go down a water slide without hitting 88 mph!
First in-person viewing
After Bri went through the consent form, she lowered my weight, height and blood pressure. Check each measurement two or three times. We went down to the lab where I felt like I had a Disney FastPass and had a small amount of blood drawn to check my A1C and thyroid, kidney and liver functions.
Second in-person viewing
It was the dreaded glucose test. The complete, fasting, two-hour glucose test. I probably won’t be drinking orange soda again! Bri and I went over a little medical history and she took my weight again. This part of the screening is what determines whether I qualify for prediabetes and therefore dictates whether I am fully eligible to participate in the study. Turns out your girl is pre-diabetic and an official participant in the SWEET study!
What is a base visit?
This visit includes an approving physician reviewing my medical history to give his blessing for my participation in the study. That doctor happens to be Dr. Neil Chappell, who is the medical monitor working on the SWEET study. He is also a fertility doctor with fertility answers and he is delightful. He is as brilliant as his bedside manner and humor.
After my initial visit, I had a full body composition scan (called a DXA) which is primarily used to track patients’ bone density, but can also provide body fat percentage data. I found out I’m 51% fat. Hello!
My last stop during this visit was with a registered dietitian to have a continuous glucose monitor placed in my stomach. I wore this device for 10 days before starting the injections and will wear it again after the study is complete. This will give the research team data to compare how my body handles my blood sugar regulation before and after the study. Periodically, Bri will request a dietary recall of the past 24 hours. This will happen every now and then during the eight month study period. While there is some accountability with this part, Bri certainly didn’t make me feel bad about my eating habits! I think of it as setting me up for success.
I hope you will continue to follow my journey as I begin the process of giving my own injections.
