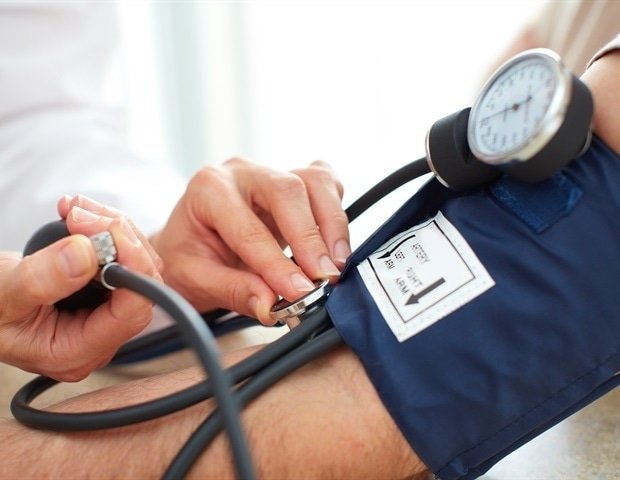
Lorundrostat, a new treatment that prevents aldosterone production by the adrenal glands, has shown clinically significant and prolonged blood pressure reductions in 1,083 patients with uncontrolled or resistant hypertension, according to the results of a Phase 3 test today.
Data from the Launch-HTN test, announced at 34th The European meeting on hypertension and cardiovascular protection shows that Lorundrostat, a aldosterone synthesis inhibitor, is a safe and effective treatment for people with uncontrolled or resistant hypertension, demonstrating consistent reductions in blood pressure in a large and different patient population. It is the largest three -phase test of a aldosterone synthester inhibitor to treat hypertension.
The results are an important milestone to provide the first targeted aldosterone synthester inhibitor for uncontrolled or resistant hypertension, which could benefit millions of people affected by conditions.
Dr. Manish Saxena, a clinical co-director of the University of Queen Maria, William Harvey Heart Center and special hypertension at Barts Health NHS Trust, is the lead researcher of the study. Said:
“Despite available treatments, more than 40% of adults with hypertension worldwide do not reach the target of blood pressure. There is a significant need to explore new treatments for hypertension and the HTN launch test concerned this need.
“Aldosterone road plays an important role in regulating blood pressure and leads to blood pressure-related complications, such as heart failure and kidney problems. In the launch-HTN test, we have explored the safety and efficacy of the Loroudotate hormones from the weakness of the seeds.
“The launch-HTN test is the largest phase 3 hypertension study with a new drug. We tried Lorundostat in a large, different population of patients hired worldwide and found that it has a good safety profile and reduces blood pressure consistently in our patient groups.”
Hypertension affects 1 in 3 adults worldwide and increases the risk of heart disease, heart attack and stroke.
30% of people with hypertension have regulated aldosterone, which means that the natural mechanism of the body to control aldosterone is disturbed. Increased aldosterone levels can cause hypertension. Lorundrostat was designed to lower aldosterone levels by inhibiting CYP11B2, the enzyme responsible for its production.
Results
The Launch-HTN test was a global, randomized, double blind, placebo-controlled phase test, which was signed up with eligible adult participants who failed to achieve the goal of blood pressure despite being in two to five anti-anti-clericals. The launch-HTN reflects the regulation of the real world for clinical doctors using the measurement of automated office blood pressure (AOBP) and allowing participants to remain on their existing medicines.
The Lorundrostat 50 mg was dosing once a day that showed clinically significant and prolonged reduction in systolic blood pressure, with a decrease of 16.9 mmHg per week (-9.1 mmHg placebo) and a decrease of 19 mmHg per week 12 (-11.7mm Placebo).